Collaborative Research
Academic research
Collaborative Research
Overview
QB3 coordinates scientists and resources across multiple UC campuses to tackle major challenges in biomedicine.

QB3 was built to unite scientists, initiate new research directions, and promote collaboration across its three campuses: UC Berkeley, UC Santa Cruz and UCSF. Each campus has strengths, be they life sciences, computation, engineering, or medicine.
The QB3 collaborative unit, spearheaded by grants coordinator Lise Barbé, leverages these strengths and resources to build a deeper understanding of complex biological systems and to stimulate the discovery of solutions to societal problems in human health and climate change. Our current focus area is neural organoids and their application in human disease and drug screening.
We continuously work with investigators at all QB3 campuses, other UC and academic institutions, and industry including startups. If you are interested in joining QB3’s Collaborative Research program, please reach out through this website’s contact form.
Current focus area: Neural Organoids
Organoids are 3D structures that self-assemble into developmentally relevant cellular models. The promise of neuronal organoids lies in their ability to more accurately model brain development, study neurodevelopmental and neurodegenerative diseases and utilize organoids as a 3D human model in drug screening. However, several roadblocks currently hinder the widespread use of organoids in these applications. Through collaborative research, QB3 aims to address some of these challenges and propel organoids to be more relevant in disease modeling and drug screening. We have currently have grant initiatives in four areas: autism, Alzheimer’s, stroke and a platform technology for vascularized brain organoids.
Neural organoids as a model for autism and other psychiatric disorders
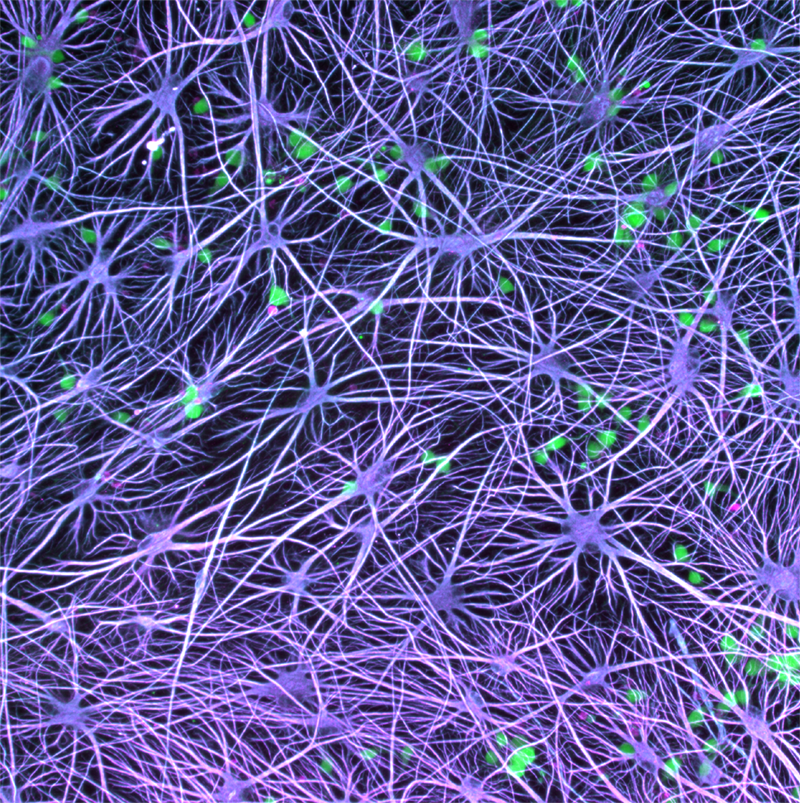
Autism and other psychiatric disorders are predominantly polygenic, and animal models often fail to replicate the diverse human disease phenotypes observed in autism and psychiatric disorders. To comprehend how different genetic causes can yield similar disease patterns, it is imperative to uncover the functions of these mutated genes. CIRM recently awarded QB3 a CIRM ReMIND-L grant, led by Alex Pollen, an associate professor of neurology at UCSF, to identify and target autism disease pathways. We will use neural organoids, patient imaging and clinical data, human brain samples, CRISPR, machine learning and gene therapy to identify and test new potential disease treatments for autism.
Neural organoids as a model for neurodegenerative diseases
Current FDA-approved Alzheimer’s disease drugs have only shown minimal effectiveness for symptom relief and have been unsuccessful to halt neurodegeneration. Alterations in microglia function have been identified in Alzheimer’s disease; however, the exact role they play in pathology remains elusive. With this effort, we aim to understand the complex interplay between microglia, pathological protein accumulation and neurotoxicity. We will use neural organoids, longitudinal imaging, OMICs, AI and gene therapy to develop effective therapeutic strategies targeting the immune response in Alzheimer’s.
Neural organoids for brain replacement in stroke
Stroke and other sudden brain insults will leave a part of the brain nonfuntional and scar tissue will replace the affected areas. The lost brain function can result in many different consequences including (partial) loss of physical, emotional, cognitive and communicative abilities and typically result in extensive rehabilitation programs. With this project, we aim to pre-assemble functional brain cells in vitro and then transplant them to the patient’s brain with minimally invasive surgery. Successful integration of the transplanted cells will result in regaining most if not all brain function that was lost.
Vascularized brain organoids to model the blood-brain-barrier and perform drug screening
Brain organoids face limitations in size and cell maturation due to the development of a necrotic core at their center. The incorporation of vascular cells alongside neuronal cells can generate open lumens and vascular channels throughout the organoids. In this project, we aim to create vascularized brain organoids with flow of nutrients and oxygen throughout the vascular structures. This approach seeks to enhance both the size of the organoids and maturation of neurons. The outcomes of this research are expected to significantly advance the utility of neural organoids in modeling the blood-brain barrier, drug screening and safety studies.
